
At around 2 Ma all the australopithecines (A. afarensis and A. africanus) had alredy disappeared. The paranthropines (P. boisei and P. robustus) survived until 1.2-1 Ma and lived together with new species of the genus Homo that are assigned to the human lineage. At least three chronospecies have been described: Homo habilis, Homo erectus and Homo sapiens, each one with an increasing dregree of brain volume and encephalization that started in A. afarensis. The first humans shared some dental traits with the australopithecines, which denotes their phylogenetic origin, while the evolution of the brain and the cranial vault significantly changed. The origin of H. habilis is linked to the climatic shift during the Pleistocene. Its survival strategies were based on the generalized use of lithic industries to obtain and process foods, which allowed a reduction of the masticatory aparathus, as well as on a complex social organization and the use of an articulated and more complex language. Scavenging and, to a certain extent, hunting for animal meat were the dietary activities, along with the gathering of wild plants and fruits. Hunting requires complex behaviours and collaboration among individuals, for which communication might be relevant. Homo habilis developed numerous adaptations for an arid and open environment. To avoid competition with predators in the open savannas, that mainly hunt at the end of the day, humans likely scavenged during the day, exposed to sun radiation and high temperatures. The progressive exploitation of open environments, especially by Homo erectus, would have resulted in the specific adaptations we have for regulation body temperature (absence of body hair, skin pigmentation, and sweating for regulating body temperature). The sex related difference in body hair distribution in modern humans is likely due to sexual selection in more recent human populations.
Body proportions also represent a means for thermoregulation, which in arid environments favours taller and more slender bodies to provide a reduced body volume (reducing the production of body heat) and an increased skin surface to maximize sweating to regulate body temperature. The bipedal locomotion reduces the extent of body skin exposed to the UV solar radiation to the head and the shoulders, which would explain the maintenance of hair in the head and, to a certain extent, in the shoulders (at least in men). Sexual selection would explain the sex related differences in hair distribution in modern humans. This evolutionary hipothesis is consistent with the body proportions of the fossil specimen WT 17000, a young Homo erectus individual, 1.6 m tall and with long and slender long bones and pelvis. The reduction in body mass with respect to body surface in humans compared to the australopithecines favours thermal regulation through sweating as well as the efficiency of the bipedal gait by having a longer leg. Other animals living in savanna enviroments regulate brain temperature by circulatory countercurrents in the mouth and do not have sweating glands and retain body hair.

Unlike Homo habilis, H. erectus shows body proportions indicative of adaptation to open environments (tall and slender bodies), maximizing skin surface and minimizing body volume. However, these adaptations might also be related to a locomotor, obliged bipedalism, even to running long distances (longer legs, sweating for body temperature regulation, reduced internal heat production). The skeleton WT 15000 (an Homo ergaster specimen) shows clear limb proportions indicative of a full bipedal (not facultative) locomotion. Its body structure is clearly distinct from that of the australopithecines and resembles that of modern humans. Some researchers consider this to be the first Homo sapiens instead of a Homo erectus. However, its brain volume is still smaller (910 cm³) than that of modern humans (1300 cm³). Whatever the case, this hominin shows many derived features (reduced facial prognathism, small teeth, reduced molars, short arms, non curved phalanges, tall pelvis, fully rotated illium, short femur head neck) that do not represent the transition from an australopithecine ancestor. In East Africa the species Australopithecus garhi was a chronological candidate (2.5 Ma) but shows australopithecine cranial and facial traits. Nevertheless, cutmarked animal bones have been described in association to the A. garhi specimen, which would suggest the use of a lithic industry. Evidence of tool use by Australopithecus begins to accumulate, but for the moment this is not sufficient to show an ancestor descendant relationship between this species and Homo. In fact, there is a significant amount of fossil remains transitional between Australopithecus and Homo that have been alternatively assigned to the species Homo habilis and, by a few researchers, to Australopithecus habilis. This discrepancy is due to the mosaic of anatomical traits of the specimens belonging to this taxon: small brain but with a developed Brocca’s area, long arms but fully opposing thumb, toe alligned with the other foot fingers, reduced facial prognathism, small teeth but with posterior molars larger than anterior ones. These anatomical traits are not homogeneous and some controversy has arised concerning the presence of 1 (Homo habilis, sensu lato) or 2 distinct species (Homo habilis, sensu stricto, and Homo rudolfensis), The two species, if accepted, share many anatomical traits:
- Brain expansion
- High supraorbital height index indicative of brain development.
- Significant increase of brain volume: average value of 640 cm³, with a minimum of 510 cm³ for ER 1813 and maximum of 775 cm³ for ER 1470 (this is the main justification of considering that the larger specimens belong to a distinct species, H. rudolfensis).
- Brain volume is 45.1% larger in H. habilis than in Australopithecus, whereas the paranthropines only show a 17% increase in brain size compared to the australopithecines.
- Increase of the size, specially in breadth, of the frontal and parietal lobes, as in later humans.
- Development of the parietal association areas 39 and 40 (Brodman) and of the prefrontal cortex.
- Medial meningieal irrigation more complex and more branched.
- Significant development of brain areas associated to language (Brocca and Wernicke areas). Australopithecus shows a rudimentary Brocca’s area and the chimpanzees lack a developed Wernicke’s area.
- Masticatory apparathus
- There are no nuchal and sagittal crests and the skull shows reduced robusticity.
- Small bizigomatic breadth indicative of reduced biting force.
- Reduced facial prognathism compared to A. afarensis and small subnasal fossa as a result of the reduction of the size of the canine roots.
- Generalised reduction of the masticatory processes and bitting muscles.
- Canines do not extend over the other teeth. The canine is small and there is no honing complex nor diastema.
- The size of the molar teeth is somewhat smaller than that of Australopithecus but their ranges geatly overlap, which makes it difficult to distinguish them.
- The M3<M2<M1 human pattern is present in many specimens.
- Premolars are square or even larger in a B-L dimension, clearly differing from the australopithecine ones that are larger in a M-D dimension.
- Other skeletal apomorphies
- Advanced foramen magnum, more efficient bipedalism
- There is still great debate on the relative length of the arms with respect to the legs. The specimen OH-62 seems to have long arms indicative of facultative bipedalism with significant arboreal capabilities.
- Derived glenoid fossa and auditory foramen.
- The hand (OH 7) is shorter than in the australopithecines but still has somewhat longer fingers than later Homo species, suggesting certain arboreal capacities.
- The escapula is more elevated than in modern humans, which resembles the australopithecines with facultative bipedalism.
- The femoral neck is short and the head is round. The short neck might be related to the increase of the birth canal for giving birth to larger brained infants maintaining bipedal efficiency in the leg.
- The foot (OH 8) shows a fully aligned toe with a strong distal phalanx, but also a robust third metacarpian (not present in modern humans).
a. Brain encephalization and language

Brain encephalization refers to the relative proportion of actual brain size to expected brain size with regard to body size. This proportion is refered to as the encefalization quotient (EQ). The EQ can be calculated in many different ways. One of the most used values is the EQ 67 (developed by H. Jerison in 1970), which uses mammal comparative samples for which the allometric relationship between body size and brain volume was 0.667. Modern humans have a EQ 67 value of 7.6, which indicates that our brain size is 7.6 larger than expected for our body size. In fact, the wolf shows an EQ 67 of 1 that indicates that its brain is the size expected for its body size, giving the allometric relationship seen in mammals. The EQ value also depends on life expectancy, animals that live longer also tend to show larger body sizes. The hominines show variable values of EQ 67: A. afarensis 3.1, A, africanus 3.4, P. boisei 3, P. robustus 3.5, H. habilis 4, H. erectus 5.5, H. erectus (Asia) 6.1, and H. sapiens 7.6. The modern chimpanzee has an EQ 67 value of 2.6. From these data, the encephalization of H. habilis is only slightly larger than that of A. afarensis. This small increase could be related to an increase of life expectancy. Nevertheless, the development of the parietal and prefrontal brain cortex is likely related to this increase in EQ. EQ values (independently of the reference sample used) can be refered to as fixed EQ value by calculating a percentage of EQ with respect to the human EQ. A relative EQ value smaller than 1 will indicate smaller encephalization than humans, an EQ value larger than 1 will indicate greater encephalization than humans. Using a relative EQ index, H. habilis shows an EQ of about 50% of that of modern humans, while the australopithecines have a 35%, the paranthropines a 45%, and H. erectus between 70 and 80% (with somewhat larger values the Asian ones). It is evident, then, that H. habilis shows a significant encephalization that distinguishes it from the australopithecines, despite some skeletal elements are still primitive.

Another relevant anatomical trait indicative of brain development is the complexity of the blood irrigation (meningeal brain arteries) of the brain. The arteries involved in brain irrigation leave visible grooves in the endocranial vault, on the inner surfaces of the cranial bones. This marks can be studied to determine the increased complexity of the irrigation of the brain in relation to brain volume increase and brain complexity (R. Saban, 1975-1984). The analysis of this anatomical trait shows that A. africanus shows simple, not branched menigeal arteries and lacks a medial meningeal branch. In the australopithecines the anterior irrigation is more developed than the posterior one, unlike in the chimpanzees that show a more developed posterior irrigation system. The paranthropines are similar to the australopithecines but already show the medial meningeal branch. Homo habilis shows a larger and better irrigated brain. The meningeal vascular system of ER 1470 already shows asymmetry in the vascularization of the hemispheres and clear anastomosis (connection between arterial branches). The left hemisphere has a simple anterior branch, a branched median branch, and a posterior branch with anastomisis with the medial branch. The right hemisphere also shows anastomosis between the anterior and medial branches, in addition to that between the medial and posterior branches. In H. erectus the branching system is much more complicated, especially in the parietal areas, already resembling that observed in modern humans.
Based on the studies of the endocranial casts of fossil hominines, Ralph L. Holloway (1995, 2000) has summarized the main differences in brain evolution between Australopithecus and Homo:
- Australopithecus afarensis (3.5-3 Ma): small volume of the primary visual striated cortex (area 17), relative increase in the parietal posterior association area, sulcus lunatus posteriorly positioned in specimen AL 162-28.
- Australopithecus africanus (3.5-2.5 Ma): small increase in brain size, mostly allometric, up to 400-450 cm³. Brocca’s area present, though absence of Wernicke’s area.
- Homo habilis (2.3-1.8 Ma): reorganization of the frontal lobe, clear presence of Brocca’s and Werknicke’s areas, significant brain assimetries, and significant increase in brain size (up to 775 cm³ in ER 1470).
- Homo erectus (1.9-1.6 Ma): moderate allometric increase in brain size to 750 cm³ in WT 15000 and to 900 cm³ in the Asian specimens.
- Homo neanderthalensis (0.25-0.1 Ma): increase in brain size to 1,200-1,700 cm³.
- Archaic Homo sapiens (0.2-0.1 Ma): extended increase in brain size up to 1,000-1,200 cm³ and refinement of the cerebral cortex as in modern humans.
- Modern Homo sapiens (<0.1 Ma): small allometric reduction of brain size and large variability in cranial capacity.
The posterior expansion of the parietal lobe is a significant trait in the hominin evolution, involving Brodmann association areas 39 and 40 (involved in the symbolic behaviour not related to emotions). These areas seem to have evolved since the differentiation of the australopithecines, and is not exclusive to the genus Homo. The anatomical trait that allows placing these areas is the sulcus lunatus, a transverse groove that divides the parietal lobe in two. The sulcus lunatus is clearly visible in all the primates except in humans, in which it is rearly positioned right at the back of the brain (caused by a significant expansion of the parietal lobe). In A. afarensis this sulcus already shows a backward position 3.5 million years ago, denoting a brain reorganization towards the human lineage. In A. africanus (Stw 505) the same posterior position is present. Holloway indicates that this posterior reorganization of the brain cortex can be due to several causes: 1) communication requirements (visual, auditory and sensory coordination); 2) greater social organization and competition (both verbal and non-verbal communication); 3) visual-spatial coordination related to the use of lithic industry or throwing objects, or to long-term memory requirements for spatial recognition and self conciousness; and 4) increase in social inteligence and sex-related differences in brain assymmetry and especialization. Brain evolution seems to have affected in the first place the parietal lobe during the transition between A. afarensis and H. habilis. Then, the rearrangement of the frontal lobe occurred in H. habilis, likely associated to the development of an articulated language. The progressive evolution of language is associated to the increase in brain size and in the increasing assimmetry and brain especialization in the later Homo species. From a genetic point of view, the developmental shifts in growth of the different cortical lobes was likely linked to regulatory genes involved in growth rates of different brain areas, such as the posterior parietal cortex (areas 7, 39 and 40) or the language associated areas.
For R. Holloway, language is a cognitive hability of the brain linked to the fabrication and use of lithic tools, strongly associated with a complex social structure based on the transmission of knowledge between individuals. This language capabilities are, in turn, associated to brain assymmetry and the lithic use of one hand (mostly the right one). Brain laterality is strongly associated with handedness preferences and the fabrication of lithic tools have shown that H. habilis was right handed, as modern humans. Homo habilis was the first hominin to have both Broca’s and Wernicke’s cortex areas, and therefore language might have been a significant adaptation for this species. Language might have appeared between 2.5 and 2 million years ago. Five sources of evidence are simultaneous in Homo habilis (meningeal development, language-related brain areas, lithic industries, increased brain volume and encephalization). These evolutionary trends were likely related to the environmental effects of the progresive climatic shift. The most ancient remains of Homo have been dated to at least 2.8-2.5 Ma and the appearence of the lithic industry is also dated to 2.5 Ma. The evidence of the neurologic capabilities of H. habilis was used by P. V. Tobias to propose that H. habilis already possessed an articulated language with a sufficient phonetic repertoire and gramatical versatility to constitute a true adaptation for survival. These capabilities certainly improved in later humans, but nowadays most researchers accept that H. habilis was the first hominin showing this suit of language-related adaptations. The question on wether language, although less developed, was present before H. habilis is yet difficult to answer. If the use of lithic industries is a requirement for language development, increasing evidence accumulates suggesting that some australopithecines could have used it (i.e. A. harhi or even A. africanus and A. sediba). From a neurological perspective, the australopithecines, and also the chimpanzee, have Broca’s brain area, but this may not be sufficient for a complex, articulated language. In addition, other types of industries (non lithic) could also require manipulative skills and brain-hand coordination, which could be also associated to gestual communication. For P. Tobias, language might have been facultative for the australopithecines but compulsory for survival in the first humans. The derived cranial traits of A. afarensis and A. sediba have been suggested as indicative of brain development in the direction of humans. However, an ancestor-descendant relationship between A. africanus and H. habilis has not been shown so far. In fact, the fossil record in East Africa provides dates for the origin of Homo in East Africa older than 2.5 Ma, whereas A. sediba is only 2 million years old. This would imply that when Homo emerged in East Africa, the australopithecines had not yet disappeared in South Africa. The initial dating of H. naledi (3-2.5 Ma) seem to support an A. africanus—A. sediba—H. naledi lineage (South African origin of Homo). However, the new dates of H. naledi (an average of 300.000 years) suggest that it is most likely related to H. erectus and that the oldest dates for Homo are found in East Africa. A truly transitional hominin is still lacking in East Africa, since A. garhi (2.5 Ma) still resembles A. afarensis in many anatomical traits. But perhaps there is no need to search for a missing link in the Australopithecus—Homo transition. As shown by the anatomical mosaic features of Homo habilis (2.8-1.6 Ma), this species is actually the missing link, likely between A. garhi (2.5 Ma) and H. erectus (1.8 Ma).
The acquisition of language was not only dependent on neurological adaptations. There are other significant evolutionary trends that evolved along with the increase in complexity of the articulated language: non-verbal communication and manipulatory habilities, auditory and visual interpretation of the surrounding environment, and muscle evolution in relation to breathing to produce sound, vocal cords, and larinx positioning (separation of swallowing and breathing). It is interesting to note that areas 39 and 40 in the brain cortex are the last to be myelinized (after birth) and the last to complete dendritic connections and neural development. This suggests that the evolution of these areas could be related to ontogenic evolutionary processes in the human lineage.
b. Diversification of the genus Homo
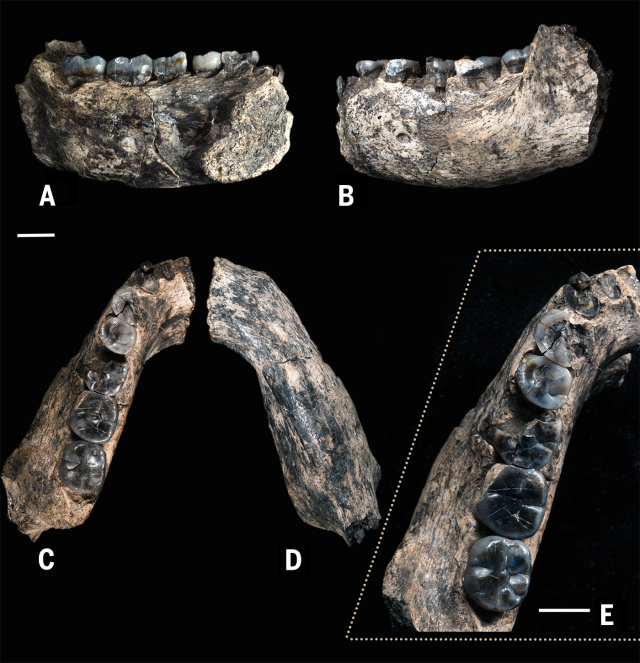
The most ancient remains that have been attributed to the genus Homo were found in 2013 at Ledi-Geraru (Afar, Ethiopia). The specimen LD 350-1 consists of a left mandibular fragment dated to 2.80-2.75 Ma. It shows both primitive traits similar to Australopithecus and derived traits seen in later Homo (Brian Villmoare et al, 2015). LD 350-1 consists in two separate fragments of a mandible with some teeth. The molar teeth are narrow and the premolars are symmetrical as in modern humans. They differ from those of Australopithecus garhi in their smaller size (the area of the lower M2 of LD 350-1 is a 64.7% of that of BOU-VP-12/130). The teeth of Ledi-Geraru are also more derived than those of Australopithecus sediba. The P4 and M3 are smaller than those of the KNM-WT 8556 mandible, dated to 3.3 Ma. LD 350-1 also shows a more primitive morphology than Homo habilis or Homo rudolfensis in the anterior portion of the mandibular corpus, though it reminds of Homo habilis in the curvature of the anterior dentition. LD 350-1 could be a late Australopithecus afarensis, with which it shows similarities in the shape of the arcade and its large overall dimension, but it clearly differs from the australopithecines, thus resembling the Homo genus, in the lack of honing complex (between the upper canine and the lower first premolar (P2), and in its flat corpus mandibularis between P3 and M2. LD 350-1 could be at the base of the phylogenetic differentiation of Homo into several distinct species.

If the dating of this specimens from Ledi-Geraru is correct, it could be a direct ancestor of the later Homo species. For simplification of the great variability of the genus, we may consider that the species Homo rudolfensis includes the Homo specimens from Ethiopia, Kenya and Malawi, whereas the species Homo habilis includes the remains from Tanzania. If these two species are accepted as distinct taxa (which may not be true), the specimen AL 666-1 (2.3 Ma) might have been the ancestral form of H. rudolfensis. This specimen shows a reduced molar size at a date of almost 3 Ma, small canine roots, and relatively deep palate and a parabolic arcade. The distinction between H. habilis and H. rudolfensis is mainly on the basis of cranial size and brain volume, much larger in H. rudolfensis. Despite these two species share many anatomical traits, many researchers consider them to be two distinct species that evolved in the same geographical region (East Afrcia) in the same chronology (2.5-1.8 Ma). The biological species concept requires interbreeding but is it impossible to test this in the fossil record. From a paleontological perspective it is fairly frequent that the discoverer of a new fossil adscribes it to a new species on the basis of a few, or even a single, distinct anatomical traits not seen in other fossil specimens. In a cladogram or a phenogram, such distinct traits would make the new fossil to branch appart from other taxa not showing them. As a general convention, fossil specimens should show a greater degree of diversity than that seen in modern humans to be considered a distinct taxon. This becomes a difficult task if chronospecies are considered (fossil specimens with differing chronologies that show a certain degree of anatomical variation through time) because it is difficult to set a specific point in time that separates the two species.
From this perspective, the human lineage can include from just one species (Homo sapiens) or up to eight different species. Most authors might accept H. habilis as a distinct human species, while some researchers include all other humans in a subspecies of H. sapiens (H. sapiens erectus, H. sapiens rudolfensis, H. sapiens heidelbergensis, H. sapiens neanderthalensis, H. sapiens sapiens). It is likely that most of the morphological variability of such human subspecies is smaller or at least similar to the variability observed in human populations, which would support their attribution to the same taxon. Moreover, molecular data have shown that inbreeding between Neandertals and modern humans took place and that we nowadays have Neandertal genes as a result of the crossed reproduction. Therefore, at least H. sapiens neanderthalensis and H. sapiens sapiens should be placed into the same species. What about Homo erectus? Did it show enough anatomical differences compared to modern humans to be assigned to a distinct species? M. Wolpoff and E. Aguirre support this view. Others, such as B. Wood and F. Grine apply a cladistic approach and divide the human lineage into up to 8 or more human species. More moderate researchers center the debate in the specific pairs: the distinction between H. habilis and H. rudolfensis and between H.ergaster and H. erectus. In both cases there is evidence that suggests that a single species should be considered in each case (H. habilis and H. erectus). We will discuss this evidence later.
Homo habilis (Olduvai, Tanzania)
The species was defined in 1964 by Leakey LS, Tobias PV and Napier JR. In 1979 Tobias indicated that H. habilis showed the evidences indicative of an articulated language for communication: 1) increased brain size, 2) brain reorganization, and 3) sistematic use of lithic tools. For Tobias, these evidenced a new level of organization that deserved a new genus: Homo. Fossil remains of H. habilis come from both East Africa (2.3-1.8 Ma) and South Africa (2.0-1.8 Ma). The main site containing H. habilis specimens is Olduvai Gorge (Tanzania, 2.1-1.6 Ma). Many specimens have been described, including OH-7 (mandible), OH 13, OH 16, OH 24, OH 65 (maxilla, 1.84-1.79 Ma).
Two alternative views have been proposed for the fossil specimens from Olduvai. They either 1) belong to a single species with great sexual dimorphism (Walker 2002, Tobias, White, Howell, Wood, Collard) or 2) they can be separated into two groups: the larger specimens (OH 7, OH 16) would represent true H. habilis, whereas the smaller ones (OH 13, OH 24) would represent late specimens of Australopithecus (Leakey & Clarke, 1971).
References
- Blumenschine, Robert J., Ian G. Stanistreet, and Fidelis T. Masao. «Olduvai Gorge and the Olduvai Landscape Paleoanthropology Project.» Journal of Human Evolution 63.2 (2012): 247-250.
- Tobias, Phillip V. «Encore Olduvai.» Science 299.5610 (2003): 1193-1194
Homo rudolfensis (Omo, East Rudolf, Hadar)

The most representative specimen of this species is the skull ER 1470, found in 1972 by Bernard Ngeneo and Richard Leakey. It shows a larger braincase and facial skeleton than H. habilis. It also shows a flatter face, less marked supraorbital torus, and larger mandible and teeth, with thicked enamel molars, similar to those of the paranthropines. In addition to the ER 1470 skull, other fossils included in this taxon are ER 1472 (a right femur), ER 1475 (proximal fragments of a right and left femurs and a tibia), and ER 1481 (a distal fragment of a left fibula). Initially, R. Leakey assigned them to Homo sp. indet. (Leakey, 1973) and later attributed them to Homo habilis. At present it is generally classified as Homo rudolfensis (and to Kenyanthropus rudolfensis by some). In 2012 Meave Leakey et al. showed a close relationship between three recent specimens (ER 60000, ER 62000 and ER 62003) and ER 1470, which supports the distinctiveness of the Homo rudolfensis species. Several sites have provided fossil specimens that have been attributed to this species: Omo (Shungura Formation, Members E and F, 2.3-2.2 MA, Ethiopia), East Rudolf (Turkana, Kenya): includes ER-1470 (a fragmented skull), ER-1590, ER-1813, Malawi (HPCR UR 501, 2.5-2.3 Ma), and Hadar (Ethiopia, Homo sp.). As indicated before, the distinction between these two species is not only geographic, but also in cranial and brain size. Nevertheless, many authors contest this taxonomy on the basis of the fossil specimen OH 65 from Olduvai. It is the last Homo specimen found at this site and has been dated to 1.84-1.79 Ma.
c. The evolution of human social behaviour

The human brain at birth is larger that that of other primates, but much smaller in relative terms to that in the adult individual. Much of the human brain growth takes place after birth. Selective pressure favouring highly encephalised brains were confronted with selective pressures limiting the width of the birth canal. Larger brains at birth were initially compensated with an increase in breadth of the birth canal by reducing the length of the femoral head neck. An increase in total pelvic width would result in an increase of the valgus angle of the femur, which would reduce the fitness of the bipedal gait. Bipedalism in the pelvis was not compatible with a larger brain size at birth. Thus, Natural Selection favoured a delay in brain development. The human brain at birth is 350 cm³ (26% of the adult size), a similar value to that in the cercopitecoidea primates (300 cm³, though 60% of adult size). However, the human adult brain size is 1,344 cm³ on the average, whereas in the cercopitecoids it is 480 cm³. Brain volumes at birth (and % of the adult volume) of the fossil hominines are unknown but some figures have been estimated: H. erectus Asia (1,043 cm³, 29%), H. erectus Asia+Africa (890 cm³, 35%), H. habilis (640 cm³, 46%), Australopithecus (480 cm³, 60%). If the size of our brain at birth would have 60% of the size in the adult, we would be born with a brain size of 810 cm³. The shift in the evolutionary trend to delay brain maturation before birth took place in the transition from Australopithecus to Homo, likely between 3 and 2.5 Ma.

Birth in the primates is mediated by hormones produced by the placental membranes corion and amnios, but it seems that the onset of childbirth is caused by an adrenal mechanism and the brain amigdala in the fetus, as in many other mammals. In humans, fetal risk induces childbirth through mechanisms favoured by natural selection, The fact that humans are born lacking a sufficient brain development suggests that the selection of such a mechanism for premature childbirth would have been advantageous. What would prevent not fully developed newborns from dying? The answer is a long and strong dependence on the mother and the aid of the social group. Selection for an efficient bipedalism forces premature birth, which causes in turn a longer infancy for brain development after birth, a longer childhood for learning, and a longer life expectancy. The demographic theory of human evolution suggests that the first humans should have based their survival on a complex social organization to assure infants to complete development after birth and survival to reproductive age. Compared to other Apes, humans show an elongation of both growth and developmnet (hypermorphosis), and a long post-reproductive phase, unique to humans. Life expectancy depends on four factors: 1) gestation, 2) intergenesic interval, 3) child-mother dependence, and 4) sexual maturation. If life expectancy becomes longer, child dependance increases and the reproductive rate decreases unless some strategies are modified to produce a demographic increase. The most significant factor that can increase population size is to reduce the intergenetic interval (the time lap between two consecutive births). This hypothesis was stated by O. Lovejoy (1981) comparing the intergenesic intervals of humans and chimpanzees.

Sexual maturity in chimps (that live up to an age of 40 years) is about 10 years of age, gestation is 34 weeks, their intergenesic interval is 5.5 years, mainly due to a long lactation period that prevents ovulation (lactation amenorrhea), and child dependance from the mother is about 6 years. If the mother dies, the infant does so, since adoption is rare among the common chimpanzee. The low rate of births in the chimpanzee makes them vulnerable to demographic withdrawals, such as mother’s death. The orangutans are also in demographic risk. They have an intergenesic interval of 7 years and the males do not get involved in taking care of the infants. Polygyneous apes are patrilineal societies in which an alpha dominant male controls the resources and the female for reproduction. But the large intergenesic interval of the Apes requires environmental stability for survival, both of the mother and the infant. The gibbons are an exception among the apes: they are monogamous (form a male-female couple during the whole life) and both females and males take care of the infants. This strategy might have been adequate for child survival (both parents take care of the infants), but also allows a decrease in the intergenesic interval since infant weaning (the end of breastfeeding) can take place sooner and ovulation also resumes sooner (postpartum amenorrhea). It is not clear if monogamy is the only factor that can induce infant care sharing. In fact, within the lemurs (a polygynic society) a female reproduces with various males and shares with them the raising of the weaned infants. Nevertheless, Lovejoy proposed that involvement of males in child care favoured a reduction of the intergenesis interval in humans. However, it is not clear if this demographic strategy was already present in the australopithecines or was exclusive to humans, and wether if it was related to the climatic shift in the Pleistocene causing the occupation of open savannas in Africa. The savanna baboon model predicted nuclear family groups in open environments for the first hominines. Could this model be applied to the first humans instead of to the first hominines? If Owen Lovejoy is correct, his demographyc hypothesis suggests several additional factors that would reinforce monogamy as a demographic strategy for increasing birth rate: continued sexual activity, not evident estrous and increased sexual attraction. Lovejoy suggests that bipedality and hair loss would have exposed sexual attributes and epigamic traits would have been favoured by sexual selection (penis size, breast size, pubic and axila hair, and hormonal secretions). A corolary of this hypothesis is that males would have no sense of fatherhood, but a continuous sexual attraction for females. It is true that sexual selection has greatly modeled many anatomical attributes in humans, but it is not clear if absence of evident estrus and secondary sexual attributes appeared as a consequence of a monogamous behaviour. In fact, the reduction of estrous in humans is likely a consequence of the modification of the reproductive patterns of humans, but it could also be in the direction of a more promiscuous society.
According to the demographic hypothesis, the reduction of the intergenesic interval in humans had to be related to the life expectancy. Tobias (1997, 1999) observed that most hominines died before arriving to the adult age. Humans have a life expectancy of 82 years on the averge (46 chimpanzees and orangutans, 32 gibbons, 41 baboons and 40 gorillas). The hominin fossil record shows that 35% of A. africanus (3.5-2.5 Ma) specimens died before the eruption on the M3 (anatomical maturity), 60% of P. robustus (1.8-1.5 Ma), and 73% of H. habilis (2.3-1.6 Ma). If over 70% of the individuals died before anatomical adulthood, they should have reproduced before that age to prevent extinction (reproductive adulthood would have been much earlier). Life expectancy in H. habilis has been estimated in less that 20 years. Tobias suggests that this figures can only be conciliated if the the first birth was right after sexual maturity and if the intergenesic interval was short, and that this had already occurred in Homo habilis some 2 million years ago. It is true that humans did not only surive to the climatic change but also expanded geographically, which requires a demographic surplus. However, a demographic increase through reducing infant mortaly before the reproductive age can be due both to increasing the intergenesic interval or through increasing parental care. The geographic expansion out of Africa, into Eurasia, of Homo erectus was likely favoured by both factors, which represented a significant shift in behaviour, with stronger social bonds, cooperation, division of labor, and sexual attraction (either monogamous or not). Sexual desire is moduled by the septum and the amigdala in the brain. In relative terms, the septum is 2.6 times larger in humans than in the chimpanzee, whereas for the medial amigdala (related to anger and agression) is 1.97 times larger in humans than in chimps. It may seem that sexual pleasure has been slightly favoured in the evolution of the brain compared to agressive behaviours. However, competition and agression are frequent behaviours in human societies and, despite such behaviours may to a certain extent be innate, the brain cortex is clearly a behavioural modulator.
The demographic hypothesis is based on a succession of evolutionary events that did not necessarily occur in this order but can be summarized as follows:
— selective pressures favoured encephalization and a delay in brain development → selective pressures shaping bipedalism limited the growth of the birth canal → child birth was premature and brain development was completed after birth → brain size at birth in H. habilis was 46% of brain size in the adult → the demographic risk favoured reduction of the intergenesic interval → parental care, including that of males, reduced mortality rates before 1 year old → changes in the brain areas related to sexual pleasure increased family bonds → social bonds and collaboration in rearing the infants increased survival → infancy and childhood increased for the transmission of a learned culture → sexual dimorphism might have reduced in a promiscuous society → estrus would be reduced and continuous sexual relationships enhanced → competition would have been mediated by social interactions → life expectancy would have increased as well as cultural transmission → further increase in brain size would have resulted in a rotary birth → assistance during birth results from complication during birth —
Behaviour, as well as morphology, is the result of the interaction of environmental stressors with the genetic code. Behaviour is in part coded by the genes. Instinctive behaviour evolved to adapt to specific environmental conditions and, thus, follows the same rules of anatomical traits. However, learned behaviour requires transmission from one generation to the next. Learned behaviour can be determinant for the survival of a species and natural selection would favour the evolution of brain capacities that would enhance learned behaviour and its transmission. Instinctive behaviour is inherited and does not require any effort to be performed, whereas learned behaviour is transmitted and requires a long time to be learned and practised skillfully. Inherited behaviour becomes inefficient if environmental conditions change, but learned behaviour can be modified quickly in front of new environmental conditions and requirements. There are 3 main learning processes in Nature:
- Trial and error: it is present in all animals, requieres multiple trials and long time for practising, but eventually it is a successful learning method and allows accumulation of experience that may be useful in the future.
- Observation and imitation: it is a faster and more efficient learning method, requieres selfconciousness and cerebral ability to copy the behaviour of other individuals, provides quick access to the behaviour learned by other individuals, but is mostly efficient when environmental conditions do not change quickly.
- Training or learning: requires a previous accumulation of learned behaviour (culture) because it is based on previous experience transmitted from one individual to another. Training can be done without an articulated language, but language is a perfect mechanism for the transmission of learned behaviour or knowledge, and especially of complex concepts and reasoning.
The transmission of learned knowledge (transmission of culture) is a unique human trait. The hability to learn is on the base of the definition of intelligence or intelligent thinking. The actions resulting from intelligent thinking can be the result of learned behaviour but it can be much more than that, becoming an original and unique behaviour, not previously learned but the result of complex interactions of culture, experience and innovation. Human behaviour is the result of a complex blend of both instinctive (genetically determined) and learned (transmitted) behaviours. Ultimately, however, transmitted behaviours are also coded by genes and inherited, at least the ability to learn them and the intelligence to process them and provide adequate responses in front of the environmental conditions.
A great number of human behaviours are influenced both genetically and environmentally. For instance, sexual behaviour and language learning have a signigicant innate component, but also require important learning skills. Sexual behaviour, food requirements and self security are likely regulated to a great extent by innate behaviours. Sexual confrontation can be regulated by dominance (as in most polygynous primates) or by social or tribal organization (as in humans). Other instincts, such as possession or territoriality can also be controlled by the rules of the society. The same happens with grooming or flirting, that in humans are behaviours highly mediated by culture, or with food sharing, compassion, solidarity or family bonds. Some researchers, such as Lovejoy think that all these constraints of the human instincts resulted in the nuclear, monogamous family structure, whereas other, such as Mayr think that a polygynic model would have been more advantageous (in part because it reduces consanguinity in a greater extent than a promiscouos model). Modern humans at present lived in societies that are more poligamous than monogamous. Finally, the concept of ethics has an uncertain classification. Aristotle defined our species as an ethic animal (unethic means deviated from the norm). However, the rules of a society are culturaly dependent. The question of wether there is only one universal Ethics or several ethical behaviours is not vain. Ethics is inextricable dependent of a complex social organization. In most primates, including apes, the interaction between individuals is determined by biological rules, such as dominance, hierarchy or social bonds and cooperation. In modern human societies, such interactions are determined by socially established rules or laws. And, in fact, different societies may have different rules and their laws may emphasize differently certain social interactions. Again, the question is, despite human cultural diversity, is there a single underlying Ethics common to all humans? And, if so, did it evolve with the origin of humans? Some would say that it arose with the advent of selfconciousness as a result of brain evolution. The sociobiologists would rather consider such complex interactions between humans, such as altruism, the result of social evolution, rather than of the selection on an individual basis. Humasn show non-adaptive behaviours, from a biological perspective, such as suicide, true altruism, or not reproducing voluntarily. These behaviours might be possible within large and complex societies because they are not determined by genes, but by culture. Cultural evolution might have substituted biological evolution to a great extent in some human societies.
References
- Eccles JC (1989) La evolución del cerebro: creación de la conciencia. Ed. Labor.
- Feibel, Craig S., Francis H. Brown, and Ian McDougall. «Stratigraphic context of fossil hominids from the Omo Group deposits: Northern Turkana Basin, Kenya and Ethiopia.» American Journal of Physical Anthropology 78.4 (1989): 595-622.
- Feibel, Craig S., Christopher J. Lepre, and Rhonda L. Quinn. «Stratigraphy, correlation, and age estimates for fossils from Area 123, Koobi Fora.» Journal of human evolution 57.2 (2009): 112-122.
- Gould SJ (1977) Ontogeny and Phylogeny. Harvard University Press.
- Leakey, Richard E. Koobi Fora research project. Clarendon Press, 1978.
- Lovejoy CO (1981) The origin of man. Science 211, 341-350.
- Oxorn H & Toote WR (1975) Human labor and birth. Appel-Century-Crofts Ed. New York.
- Rosenberg K & Trevathan W (1996) Bipedalism and human birth: the obstetrical dikema revisited. Evol Anthropol 4(5), 161-168.
- Schrenk, F., et al. «Early hominid diversity, age and biogeography of the Malawi-Rift». Human Evolution 17.1-2 (2002): 113-122.
- Short RV (1976) Evolution of human reproduction. Proc Royal Soc London B 195: 3-24.
- Tobias PV (1981) Evolution of the human brain, intellect and spitrit. Adelaida University Press.
- Tobias PV (1997) Evolution of the brain size, morphological restructuring and longevity in early hominids. In (Dani SU et al. Eds.) Principles of neural aging.
- Tobias PV (1999) Dating, demography, ecology and encephalization in relation to human evolution. South African J of Sci 95, 189-193.
- Walker, Alan, and RICHARD E. Leakey. «The hominids of East Turkana.» Scientific American 239.2 (1978): 54.
- Witzhu VJ (1997) Flexibility and paradox: the nature of adaptation in human evolution. In (Morbeck et al Eds.) The evolving female. Princeton University Press.
- Wood, B. A. «Koobi Fora Research Project IV: Hominid Cranial Remains from Koobi Fora.» Oxford: Oxford University Press (1992)‘Origin and evolution of the genus Homo’, Nature 355 (1991): 783-90.
⇐ previous – next ⇒